- Return to Article Of The Month index
Soil acidity and its relationship with nutrient use efficiency
February, 2013
Maria L. Silveira, Soil and Water Science Program,
UF/IFAS Range Cattle Research & Education Center
Soil pH is the most commonly measured soil property. It is also one of the most useful and informative soil parameter because of its relationship to many aspects of soil fertility and plant growth. Despite its importance, the implications of inadequate soil pH on forage response, particularly nutrient use efficiency, is often overlooked. The main reason for this is perhaps, the relatively greater forage response to fertilization in comparison to lime application. In other words, although liming typically does not result in forage yield increases as high as nitrogen fertilizer, maintenance of adequate soil pH is essential for long-term persistence and productivity of grass pastures in Florida. Soil acidity often creates favorable conditions for pest and disease infestations which may impact pasture longevity.
What does soil acidity mean?
Soil pH is a property that describes the relative acidity or alkalinity of the soil. The pH scale of soils ranges from 1 to 14. Lower the soil pH typically means greater the acidity (Figure 1). Soils with pH less than 5 are considered strongly acid, while values of 5 to 5.5 and 5.5 to 6.5 are considered moderately and lightly acid, respectively. Soil pH range of 6.6 to 7.2 is considered neutral, 7.3 to 8.2 is slightly alkaline and above 8.2 is moderately to strongly alkaline. The pH of most inorganic soils in Florida is around 4 to 6. Soils in Florida are acidic because of their parent material as well as the environmental conditions (i.e., intensive rainfall) in which these soils were formed. Agricultural practices, such as fertilization and crop removal, also contribute to increased soil acidity. Since pH is measured using a logarithmic scale, a decrease of 1 unit of pH results in a 10-fold increase in acidity, so small changes in pH values can have important implications in terms of nutrient availability. 
Soil acidity affects plant growth
Negative effects of soil acidity on forage growth are generally not caused by a single factor, but several that affect normal plant development. The main factors that typically affect plant growth in acidic soils includes toxicity of hydrogen ion (H+), aluminum, and manganese as well as deficiency of essential nutrients such as phosphorus, magnesium, and micronutrients. Plant species and genotypes within species differ widely in tolerance to soil acidity. The majority of the warm-season grasses cultivated in Florida are more tolerant to soil acidity than cool-season grasses or legumes. Bahiagrass is particularly tolerant to low soil pH, whereas perennial peanut require relatively higher pH levels. Despite the crop tolerance to moderate acid conditions, nutrients use efficiency can be affected by soil pH.
Neutralizing soil acidy
A large number of methods for determining lime requirement are commonly used and each state in the USA has its own method. These methods indirectly take into account properties that affect soil buffering capacity such as soil texture, organic matter concentrations, and the initial soil pH value. Most methods are based on equilibrating the soil with a buffer solution. The Adam-Evans test is the standard lime-requirement test for Florida. This test is only valid when soil pH is < 6.0. Alabama, Florida, Georgia, Tennessee, South Carolina, and Virginia also use the same method for lime requirement determination. Soils with high buffering capacity (high clay and organic matter concentration) require more lime to reach the target pH than soils of similar pH and low buffering capacity. In general, sandy soils have lower buffering capacities and thus, require less lime to raise the pH than loamy soils. However, soils with lower buffering capacities will require more frequent lime applications to maintain pH.
The most common liming materials are dolomitic and calcitic limestone, calcium and magnesium oxide, slag, sludge, and wood ashes. Since the solubility of these materials is typically very limited, it is important they be applied 3 to 6 months prior to seeding or fertilization. The reactions that take place in the soil when lime is applied will only occur in presence of water. If soil moisture is not adequate, the positive effects of lime in neutralizing soil acidity will be very limited. The quality of the lime material is expressed in terms of effective calcium carbonate equivalence (ECCE). Lime recommendations are generally based on lime material that has 100% ECCE. If a different material is used, the rate should be adjusted by dividing the recommended rate by the actual ECCE of the material. The ECCE of lime materials is affected by two main factors: 1. Fineness of the material or particle size, and 2. Chemical purity. The physical composition of liming materials is defined by the percentage of the materials that pass through 10-, 60-, and 100-mesh sieves. Finely ground materials normally neutralize soil acidity faster than coarse liming materials. Materials that contain a range of particles may be desirable when soil pH is not required to be increased in the short-term. Small particles will react rapidly and the coarser particles will neutralize acidity over time. Besides the fineness of the material, the chemical composition and percentage of impurities will also impact the effectiveness of liming materials. The purity of the liming material is measured by the “Calcium Carbonate Equivalence” or CCE. A material with CCE of 100% is equivalent to pure calcium carbonate.
Soil pH and nutrient availability
The optimum uptake of most nutrients occurs at a soil pH near neutral (Figure 2). The availability of most macronutrients (nitrogen, phosphorus, potassium, sulfur, calcium, and magnesium) decreases as soil acidity increases. Therefore, application of lime to moderately acid soils tends to increase the availability of these nutrients. On the other hand, most micronutrients decrease their availability as soil pH increases. Under these conditions, nutrient deficiencies may occur in response to excessive lime application. This “lime induced” deficiency is particularly important in Florida where the majority of the inorganic soils present low buffering capacity and, therefore, can change the pH easily in response to liming.
The most important implication of the relationship between soil pH and nutrient uptake efficiency is that fertilizer use and crop response are expected to change as a function of soil pH. In acid soils, liming improves soil pH and thus, increases nutrient availability and use efficiency.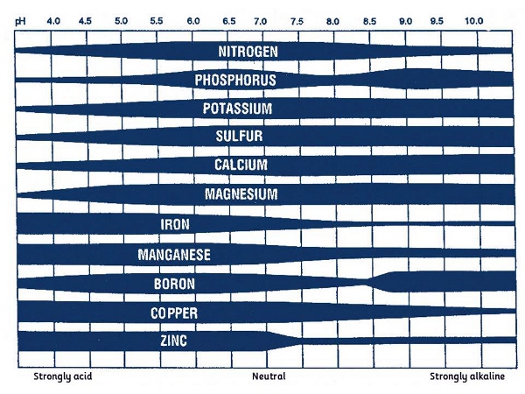
Figure 2. Nutrient availability as affected by soil pH.
Nitrogen – Soil pH influences soil microbial activities that affect nitrogen availability in soils. However, because most of these microbial transformations occur over a rather wide range of soil pH, significant inhibition because of soil acidity can only occur in strongly acid soils (< pH 5). Because lime is typically surface-applied in pastures, significant amounts of nitrogen from ammonium and urea fertilizers may be lost by volatilization if fertilizer is applied immediately after the lime application. Therefore, it is important to allow at least 3 months for the lime material to react with the soil before nitrogen fertilization.
Phosphorus, potassium, and sulfur – Availability of phosphorus, potassium, and sulfur generally increases in response to lime application. Similar to nitrogen fertilizer, application of phosphorus fertilizer immediately after liming may reduce phosphorus availability because phosphorus may form insoluble compounds with calcium present in the lime material.
Calcium and magnesium– Because lime materials often contain calcium and magnesium, the availability of these nutrients is increased.
Micronutrients – liming-induced deficiencies of zinc, manganese, and copper have been extensively cited in the literature for a variety of crops but reports specifically on forages are scarce. Although the availability of most micronutrients decrease as the soil pH increases (with exception of molybdenum, which appears to be less available under acidic pH), micronutrient deficiency in Florida pastures is rare. Boron deficiency may be a problem in alfalfa and other cool-season legumes. Molybdenum deficiencies in forage legumes have been reported when soil pH is above 6.0.
The aim in managing soil acidity is to reach a target pH range that optimizes crop performance and nutrient availability. Soil testing remains the best management tool for monitoring soil nutrient and pH levels. Soil should be tested at least every 3 years to ensure that optimum soil fertility conditions are met. Although liming can be done at any time of the year, it is important to apply lime at least 3 months prior to spring fertilization to allow time for the material to react in the soil. If you need further assistance with proper soil sampling and interpretation of soil test results, consult with your local county agent or other university personnel.

